
The FDA has warned the public against using smartwatches and smart rings that claim to measure blood glucose.
In a safety communication issued on 21 February, the FDA wrote:
“The U.S. Food and Drug Administration (FDA) is warning consumers, patients, caregivers, and health care providers of risks related to using smartwatches or smart rings that claim to measure blood glucose levels (blood sugar) without piercing the skin.”
It continued: "The FDA has not authorized, cleared, or approved any smartwatch or smart ring that is intended to measure or estimate blood glucose values on its own."
A quick trawl of Amazon reveals a host of no-name smartwatches that promise the tracking of blood glucose and other cutting-edge metrics.
Amazon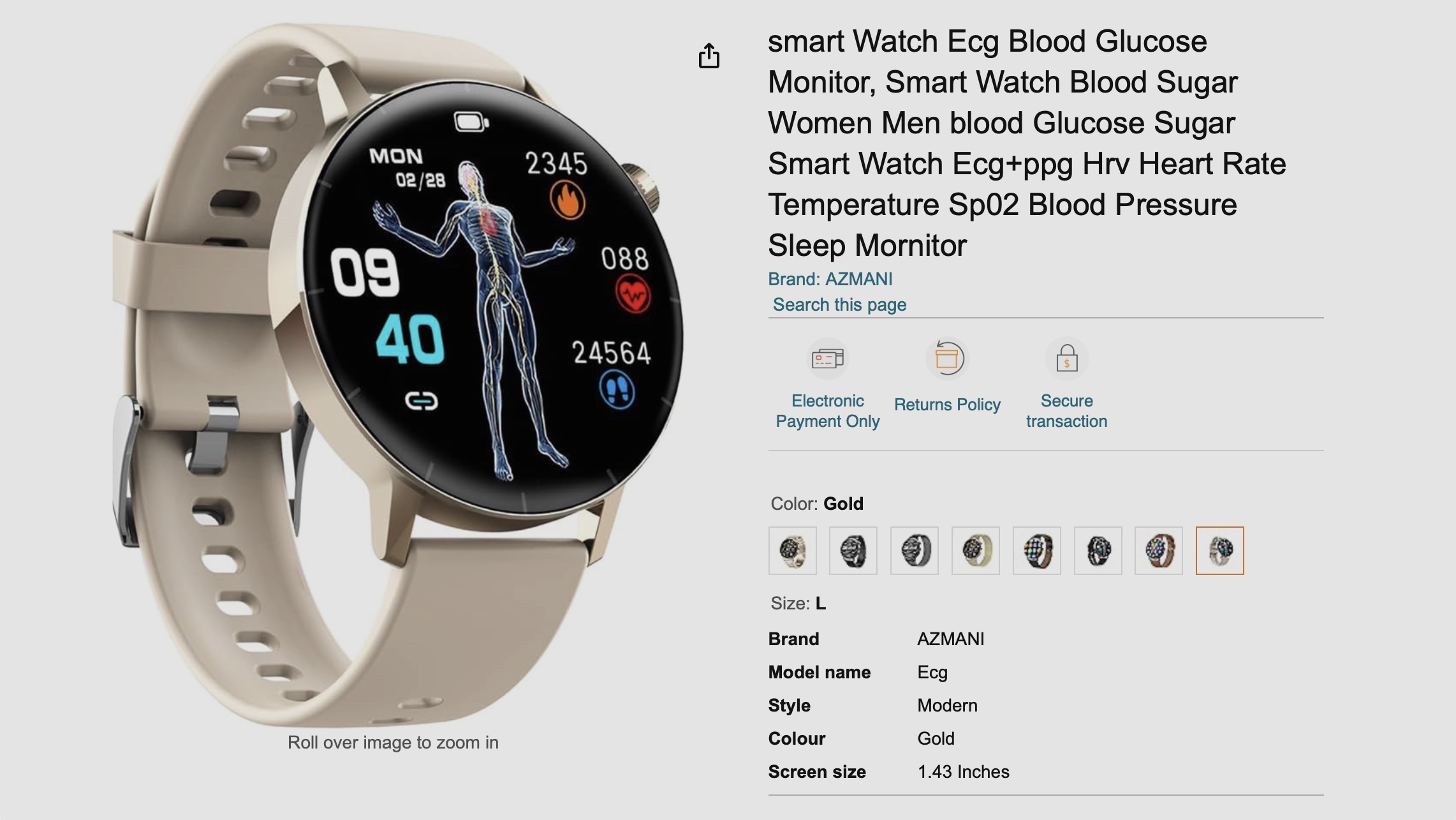
And it's not just limited to shady Chinese resellers.
The smartwatch myMonX claims to track blood glucose, without being clear that it’s AI estimated.
These implementations are clearly stated as not for medical use. But there's nothing to stop people with insulin requirements from taking the readings literally and medicating themselves accordingly.
The FDA report notes that this kind of mistake could lead to serious outcomes, including death, within hours.
Providing incorrect readings to users could lead to changes in eating habits that could be detrimental to health.
Continuously providing lower-than-accurate blood sugar readings could lead to pre-diabetics not adopting healthier choices, for example.
It feels like a precursor to the storm of glucose wearables coming down the pipe in the next few years.
And the FDA is sounding a warning shot that it takes this area extremely seriously.
How we test
